Method Qualification
Method Qualification. Studies carried out to analyze the quality of an analytical method and to check the "readiness" of a method for validation. Method Qualifications are structure in three distinct levels.
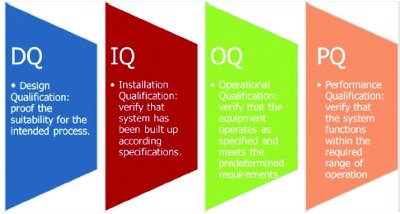
Method Qualification - Cytovance can qualify analytical methods, to be used for in-process or release testing, according to parameters described in the ICH guidelines Validation of Analytical Procedures.
We have also included a qualification of citations following the Shepard's method.
Method qualification has emerged as the typical means of filling the gap for assessing the suitability of analytical method performance at earlier stages of product development. Laboratory and method qualification for dietary supplements is critical for meeting GMP's. Method Qualification is the documentation that your analytical method yields results with the required accuracy and precision.







0 Response to "Method Qualification"
Posting Komentar