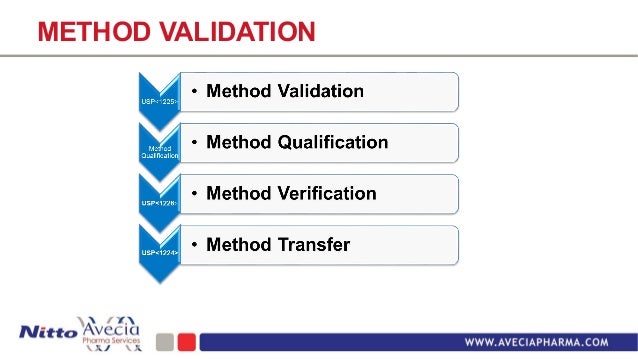
Method Qualification Vs Validation
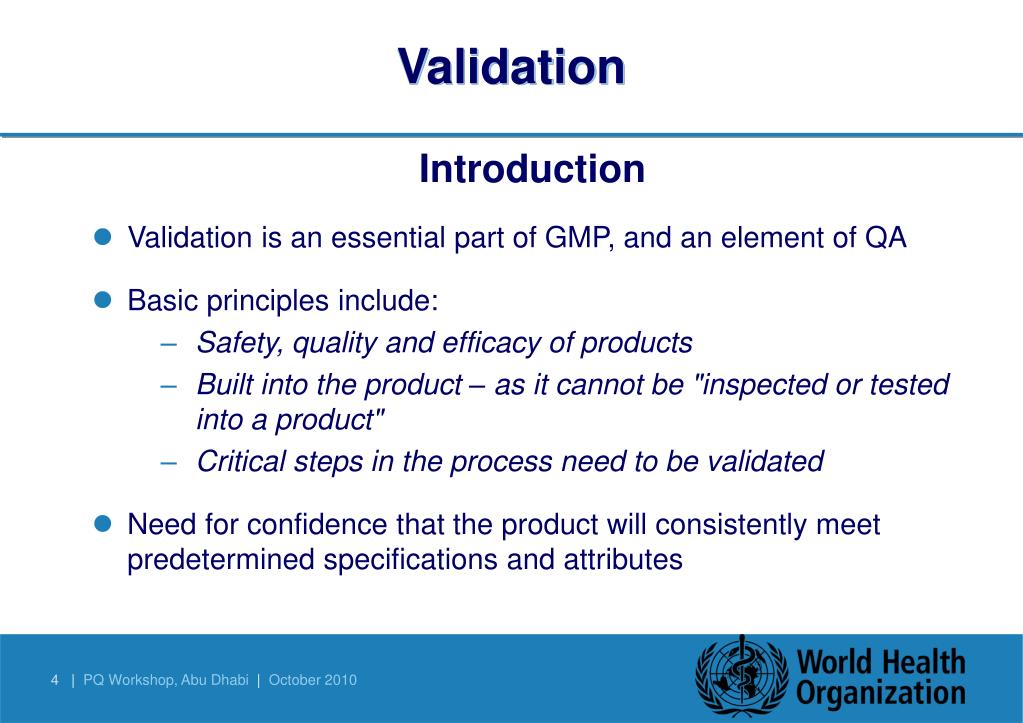
Method Qualification Vs Validation. This blog explains the difference between these two In short, validation is the documented objective evidence that a specific process, procedure, or activity will consistently produce results meeting. Validation - What Does That Mean Now?

To help ensure that you understand your system's limitations and your.
A: Method validation involves conducting a variety of experiments that focus on performance elements of Qualifications?
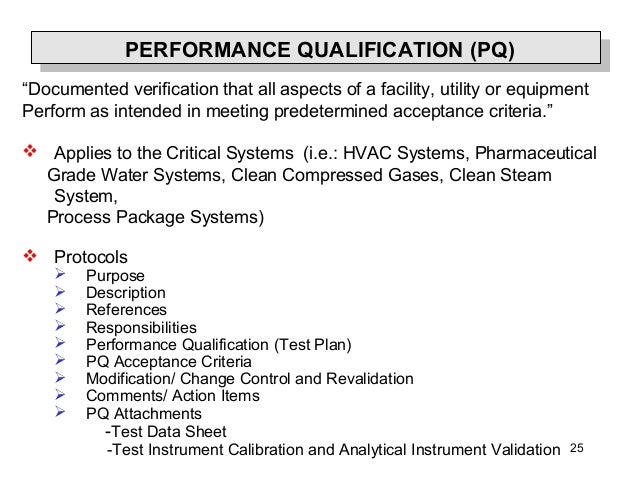
Qualification is part of validation, but the individual qualification steps alone do not constitute process validation." Validation and qualification are not one-time events: The validated state must be maintained. › Get more: Qualification vs validation equipmentView Economy. Short discussion about difference between Validation and Qualification. Industry Guidance for Qualification and Validation For analytical scientists, there is clear guidance on analytical and/or equipment qualification versus analytical and/or equipment validation. "Validation" is generally described as the process of establishing documented evidence that provides a high.





0 Response to "Method Qualification Vs Validation"
Posting Komentar